


Quick Links:


All labs working with biological materials are required to complete a biological registration with HSE. This registration includes:
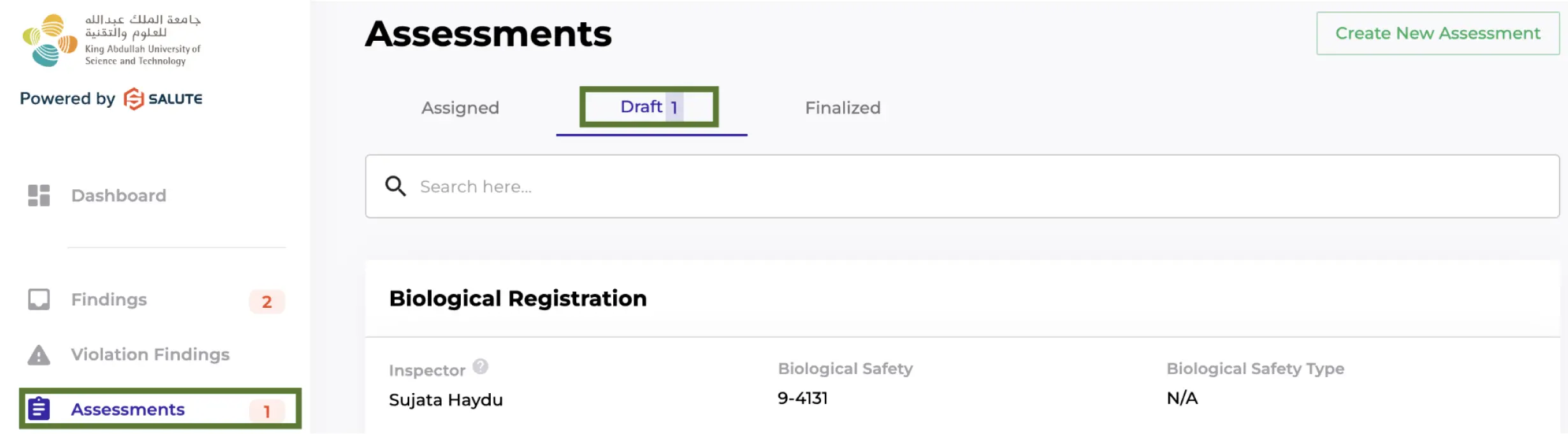
This will need to be reviewed by Principal Investigators on an annual basis using Biological Registration assessment in Salute. It is a simple checklist to declare a laboratory’s level of involvement in research with biological materials: that the Principal Investigator has active IBEC or IACUC protocols, and if not, a brief summary of laboratory activities with biological materials.
Biological Registration assessment will be posted annually (or upon change) in Salute for Principal Investigators to review and finalize. When posted, the Biological Registration assessment can be found in the PI's Salute Community Portal under Assessments - Draft.