



Quick Links:


The use of corrosive, pyrophoric, oxidizing, toxic, or highly toxic gasses present many additional hazards other than fire, asphyxiation, or oxygen enrichment. Exposure to some gases may present serious hazards to unprotected personnel.
The purposeful creation of engineered nanomaterials is a relatively recent discovered technology. The ability to create very small scaled structures (<100 nanometers) has brought forward the increased ability to perform tasks much more efficiently due to nanomaterials increased surface area compared to the same bulk material. Exposure to some gases may present serious hazards to unprotected personnel.

Guidelines for Potentially Explosive Experiments
Some chemical compounds can be highly energetic/explosive, thus presenting significant hazards. Some classes of compounds can be sensitive and decompose into a violent release of energy and gas with the slightest input of energy (heat, light, friction, shock).
The purposeful creation of engin To prevent fires in labs, flammable and combustible liquids require special precautions for their storage, handling and use. The National Fire Protection Agency (NFPA) has developed guidelines for the safe storage and use of flammable and combustible liquids in laboratories. ered nanomaterials is a relatively recent discovered technology. The ability to create very small scaled structures (<100 nanometers) has brought forward the increased ability to perform tasks much more efficiently due to nanomaterials increased surface area compared to the same bulk material.

A flammable solid is defined as a “solid, other than a blasting agent or explosive, that is liable to cause fire through friction, absorption of moisture, spontaneous chemical change, or retained heat from manufacturing or processing, or which can be ignited readily and when ignited, burns so vigorously and persistently to create a serious hazard.” An example of a flammable solid is gunpowder.

Under the IATA hazard class system, flammable solids are listed as hazard class 4. Flammable solids are further broken down into three subcategories:
Many of the same principles for handling and storing flammable liquids apply to flammable solids. Always keep flammable solids stored away from oxidizers, and away from heat or ignition sources such as radiators, electric power panels, etc.

Spontaneously combustible materials are also known as pyrophorics. These chemicals can spontaneously ignite in the presence of air, some are reactive with water vapor, and most are reactive with oxygen. Two common examples are tert-butyllithium under hexanes and white phosphorus.
In addition to the hazard of the spontaneously combustible chemical itself, many of these chemicals are also stored under flammable liquids. In the event of an accident, such as a bottle being knocked off a shelf, the chemical can spontaneously ignite and a fire can occur.
Extra care must be taken when handling spontaneously combustible chemicals. When transporting these chemicals, it is best to use a bottle carrier and carts.


Dangerous when wet compounds react violently with water to form toxic vapors and/or flammable gases that can ignite and cause a fire. Please note, attempting to put out a fire involving dangerous when wet materials with water will only make the situation worse. Special “Class D” fire extinguishers are required for use with dangerous when wet compounds. Common examples include sodium metal and potassium metal.
It is important to note that any paper toweling, gloves, etc., that have come into contact with these materials need to be quenched with water before disposing of them in metal trash cans in order to prevent potential fires.
If you are using dangerous when wet compounds and do not have a Class D fire extinguisher present, then please contact the Help Desk at 959.
Oxidizing agents are chemicals that bring about an oxidation reaction. The oxidizing agent may:
The intensity of the oxidation reaction depends on the oxidizing-reducing potential of the material involved. Fires or explosions are possible when strong oxidizing agents come into contact with easily oxidizable compounds, such as metals, metal hydrides or organics. Because oxidizing agents possess varying degrees of instability, they can be explosively unpredictable.

An oxidizer is defined as “a chemical other than a blasting agent or explosive that initiates or promotes combustion in other materials, thereby causing fire either of itself or through the release of oxygen or other gases.” Under the IATA hazard class system, oxidizers are listed as hazard class 5.1 and organic peroxides are listed as hazard class 5.2. An organic peroxide is defined as “an organic compound that contains the bivalent –O-O- structure and which may be considered to be a structural derivative of hydrogen peroxide where one or both of the hydrogen atoms have been replaced by an organic radical.” Oxidizers and organic peroxides are a concern for laboratory safety due to their ability to promote and enhance the potential for fires in labs.
As a reminder of the fire triangle (now referred to as the fire tetrahedron), in order to have a fire, you need:
Oxidizers can supply the oxygen needed for the fire, whereas organic peroxides supply both the oxygen and the fuel source. Both oxidizers and organic peroxides may become shock sensitive when they dry out, are stored in sunlight, or due to contamination with other materials, particularly when contaminated with heavy metals. Most organic peroxides are also temperature sensitive. As with any chemicals, but particularly with oxidizers and organic peroxides, quantities stored on hand should be kept to a minimum. Whenever planning an experiment, be sure to read the MSDS and other reference documents to understand the hazards and special handling precautions that may be required, including use of a safety shield. Also be aware of the melting and autoignition temperatures for these compounds and ensure any device used to heat oxidizers has an over-temperature safety switch to prevent the compounds from overheating. Laboratory staff should be particularly careful when handling oxidizers (especially high surface area oxidizers such as finely divided powders) around organic materials. Avoid using metal objects when stirring or removing oxidizers or organic peroxides from chemical containers. Plastic or ceramic implements should be used instead. Laboratory personnel should avoid friction, grinding, and impact with solid oxidizers and organic peroxides. Glass stoppers and screw cap lids should always be avoided and plastic/polyethylene lined bottles and caps should be used instead.
If you suspect your oxidizer or organic peroxide has been contaminated (evident by discoloration of the chemical, or if there is crystalline growth in the container or around the cap), then dispose of the chemical as hazardous waste or contact HSE at hse@kaust.edu.sa for more information. Indicate on the hazardous waste tag that the chemical is an oxidizer or organic peroxide and that you suspect contamination.

Exposure to even small amounts of highly toxic and toxic chemicals used in the research laboratory can cause serious injury and even death. Because of their high acute toxicity, all handling of toxic compounds must be controlled through good experimental design, active supervision and thorough training on the lab specific Standard Operating Procedures (SOPs) for these materials.
Failure to follow proper handling procedures can expose research and/or support staff to dangerous chemical compounds. Before working with highly hazardous toxic chemicals, lab workers should be trained on the proper lab specific procedures (SOPs). The written SOPs must include detailed information on how to do the work safely. The safety information in this document can assist with the development of an SOP specific to the laboratory procedure.
Examples of Highly Toxic/Toxic Chemicals Used in Research Labs:s§ Highly Toxics: Solid or liquids having a lethal dose (LD50) of less than or equal to 50 milligrams per kilogram body weight. Generally these compounds have a NFPA Health Hazard rating of 4. Examples include: arsenic trichloride, sodium cyanide, sodium azide, dimethylmercury, and di-isopropyl fluorophosphate.
Gloves carefully selected on the basis of the hazard, shall always be worn when handling these chemicals. Be sure to review glove permeability ratings provided by glove suppliers. Double gloving is strongly encouraged while handling toxic chemicals. Wash reusable gloves before removal. Carefully remove gloves to avoid contaminating hands. Wash hands and arms immediately after working with these materials.
If the chemical fume hood is used properly, respiratory protection devices are not likely necessary. If you think you need to wear a respirator, contact HSE at hse@kaust.edu.sa for more information. Dust masks provide no protection from vapors or gases.
Corrosive materials are substances that “cause irreversible tissue damage to human tissue”. Human tissues to be damaged from corrosive materials are not only limited to eyes and skin but also to the respiratory tract through inhalation and gastrointestinal tract through ingestion.

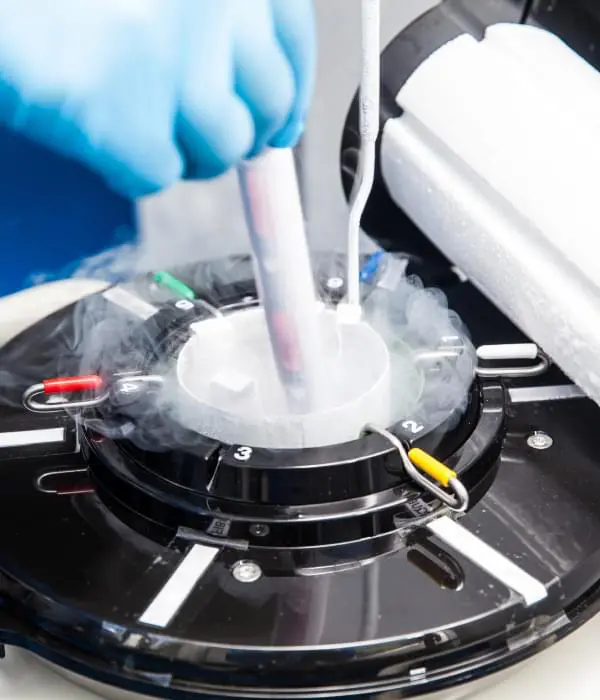
The safe handling of cryogenic liquids involves understanding the unique properties of these materials and ensuring that appropriate safety precautions are taken at all times.