



Quick Links:


Perchloric acid (HClO4) is a colorless, odorless, fuming liquid that is miscible with water, extremely corrosive and a strong oxidizer. If your lab
inventory includes perchloric acid, follow these guidelines to protect yourself from injury. Persons working with perchloric acid should be thoroughly familiar with general guidelines for the safe handling of hazardous chemicals supplemented by additional precautions particular for this chemical.
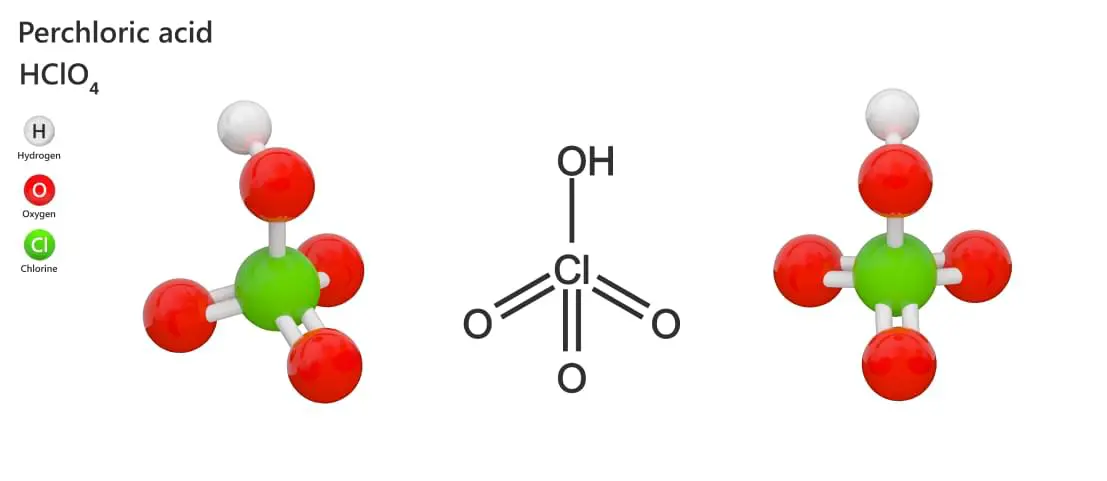
Perchloric acid is not only a highly corrosive material it is a strong oxidizer as well. Therefore not only will one need to follow the basic precautions of working with corrosive materials as detailed and outlined in the preceding pages but one will need to follow basic guidelines of working with oxidizers as well. However, perchloric acid only acts as an oxidizing agent when heated or in the anhydrous state (>85%), which then it can be explosive when in contact with organic matter.
Perchloric acid does readily react with metals to form potentially explosive metal perchlorate salts. This is why if perchloric acid is to be used on a regular basis in fume hoods, these fume hoods need to be specially designed and have a wash down feature. Perchloric acid fume hoods are specially designed with this wash down feature as well as other features such as a collection basin and non-reactive furniture (e.g. plastic, ceramic, etc) to reduce metal-perchloric acid interaction. Perchloric acid fume hoods are rare and mostly found in institutions where Geology type work is done – Perchloric acid is heated and used for rock and mineral digestions.
(in addition to the already stated for Corrosive Materials)


Heating of perchloric acid or perchloric acid reactions that involve heat shall NOT be conducted in a general purpose fume hood. A special perchloric acid hood is needed for these experiments.
Use of perchloric acid (<72%) at ambient temperature may be conducted in a general purpose fume hood if the following procedures are followed:
Heating of perchloric acid or perchloric acid reactions that involve heat shall NOT be conducted in a general purpose fume hood. A special perchloric acid hood is needed for these experiments.
In the event of skin contact, immediately wash with soap and water and remove contaminated clothing. Seek medical attention immediately.
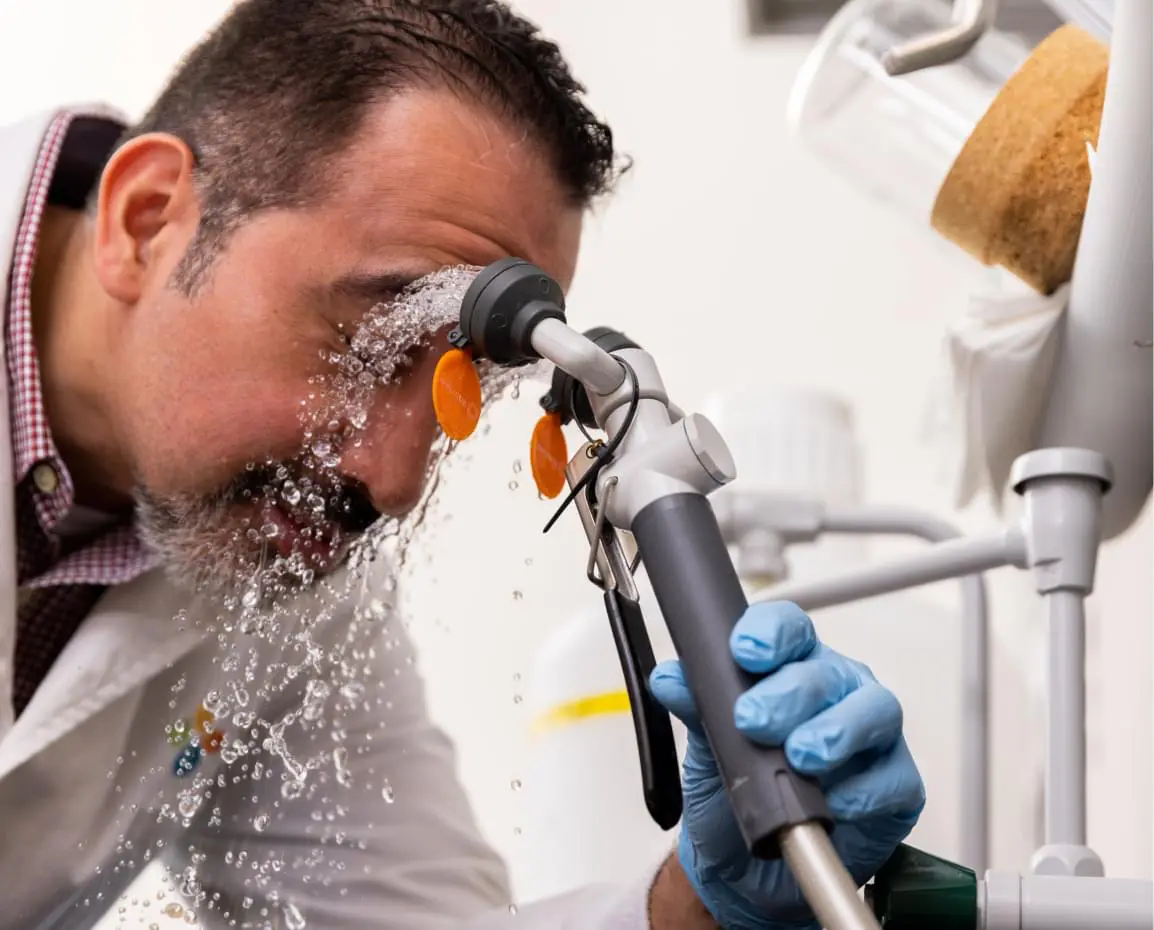
In case of eye contact, promptly wash with copious amounts of water for a minimum of 15 minutes (lifting upper and lower lids occasionally) and obtain medical attention.
If perchloric acid is ingested, obtain medical attention immediately. If large amounts of this compound are inhaled, move the person to fresh air and seek medical attention at once.

Excess perchloric acid and waste material containing perchloric acid should be placed in a glass reagent container and labeled as Hazardous Waste.